

LETTER TO HEALTH FREEDOM SUPPORTERS
FROM THE ALLIANCE FOR NATURAL HEALTH
By Dr Rob Verkerk, Executive Director, Alliance for Natural Health
Facing the challenge
There can be no denying that health freedom rights are being seriously challenged and eroded in many parts of the world.
This is no accident or coincidence. It is the result of pressures by corporate interests and agreements by governments and implementing authorities. On the face of it, the regulators justification for increasingly restrictive legislation seems quite reasonable. We are told: let us harmonise legislation in different parts of the world to facilitate trade between different nations and let us regulate more carefully substances that might pose some risk to human health. These arguments, however, begin to disintegrate when one looks at them more closely.
Firstly, the only problems with free trade occur when particular governments (e.g. Germany, France, Sweden, Greece, etc.) impose very restrictive national legislation themselves, so that those companies operating from within a more liberal legislative environment are not able to trade. So why impose trade restrictions if the goal is to facilitate trade? Why must we always be subservient to the lowest common denominator?
Secondly, there is absolutely no evidence to show that nutritional supplements pose any type of serious risk to human health when compared with other products. In fact, the evidence demonstrates quite the reverse. Nutritional supplements are statistically the safest group of products that we place in our mouths – data from the US and New Zealand showing clearly that food is many times (often hundreds of times) more likely to cause adverse reactions than nutritional supplements. And why do the authorities responsible for safety issues never look at risk:benefit equations for dietary supplements? As a society we seem happy to accept the motor car with all its inherent human and environmental risks – because it has very distinct advantages – how about applying the same logic to dietary supplements? Clearly, it is not difficult to see just how disproportionate is the EU legislation facing dietary supplements.
2002 was a very important year for health freedom in Europe, and indeed the world. We saw the passing of the framework Food Supplements Directive into European law (see Table 1). This directive, if left to the devices of the European Commission, the Council of Ministers and many of its corporate supporters, will create very real problems for the innovative sector of the dietary supplement industry. It could also massively reduce the ability of the public at large to gain access to supplements that are now being shown increasingly through numerous scientific studies worldwide to be essential to the maintenance of good health.
2002 also saw the First Readings of the pivotal Pharmaceuticals (Medicines) Directive (PD) and the Traditional Herbal Medicinal Products Directive (THMPD) (see Table 1). The PD is being used by the Commission to sweep up so-called “borderline products”. These are basically regarded as products that might have a significant effect on physiological processes in the body but are at the same time not conventional drugs. They might work via a nutritional rather than a primarily pharmacological (drug-like) route. So-called “borderline products” represent the classic safe, effective and innovative supplement – and the rash of new legislation will close the door on many of these products unless there is substantial intervention in the law-making process.
It is critical that those concerned with health freedom understand the importance of pushing though amendments to the PD that will prevent many supplements from being categorised as medicinal products. This is one of the key tasks of the ANH (see Table 1).
Table 1. Summary of key European legislation affecting dietary supplements
|
|
Food Supplements Directive (FSD)
|
Pharmaceuticals Directive (PD)
|
Traditional Herbal Medicinal Products Directive (THMPD)
|
|
Status
|
Passed into EU law 10 June 2002 First | First Reading 23 October 2002 | Reading 21 November 2002 |
|
Impact
|
Limits ingredients (nutrient sources) and maximum dosages. Framework structure; only applies to vitamins and minerals at present, will cover other nutrients in future. Full impact will not be felt until 2005 - 2009. Is likely to omit 285 nutrient sources that are currently used in Europe. Improved prospects for trade between European countries. |
All dietary supplements that are not controlled under FSD will be controlled under PD. A drugs regime would therefore apply and this would not be affordable for many non-pharmaceutical-owned supplement manufacturers. | A derogation of PD which allows fast-track legislation for eligible herbal products. The number of products caught will ultimately depend on PD definition of a medicine. Allows for improved labelling and quality control of medicinal herbs. |
|
Potential for improvements to Directive
|
Can affect implementation in EU member states through national authorities. Can influence maximum permitted levels of nutrients. Consider challenging entire legality of directive. |
Can alter the definition of a medicine, as well as scope of directive, to ensure that most dietary supplements cannot fall under PD. Can positively exclude non-medicinal food supplements, herbs and cosmetics. |
First Reading amendments allow for combinations of herbs and nutrients, but make ineligible herbs that have less than 10 years use in EU. Can promote amendments for Second Reading which allow traditional (e.g. 30-year) use from outside the EU with evidence from a competent authority. |
|
Summary of ANH achievements in 2002
|
Mounted Brussels and UK-based campaign which helped to almost block FSD’s passage through Second (final) Reading in EU Parliament. | Lobbied to ensure tabling of critical amendments on definition of a medicine, scope of directive and exclusions. All amendments successfully voted for at First Reading. | Lobbied to help ensure major amendments were supported. Key amendment on non-EU traditional use was lost in pre-First Reading vote but successfully re-tabled by European Liberal Democrats at First Reading plenary. Although it again failed, it can be re-tabled at Second Reading. |
Framework structure; only applies to vitamins and minerals at present, will cover other nutrients in future. Full impact will not be felt until 2005 - 2009. Is likely to omit 285 nutrient sources that are currently used in Europe. Improved prospects for trade between European countries. All dietary supplements that are not controlled under FSD will be controlled under PD. A drugs regime would therefore apply and this would not be affordable for many non-pharmaceutical-owned supplement manufacturers. A derogation of PD which allows fast-track legislation for eligible herbal products. The number of products caught will ultimately depend on PD definition of a medicine. Allows for improved labelling and quality control of medicinal herbs.
Potential for improvements to Directive Can affect implementation in EU member states through national authorities.
Can influence maximum permitted levels of nutrients. Consider challenging entire legality of directive.
Can alter the definition of a medicine, as well as scope of directive, to ensure that most dietary supplements cannot fall under PD.
Can positively exclude non-medicinal food supplements, herbs and cosmetics.
First Reading amendments allow for combinations of herbs and nutrients, but make ineligible herbs that have less than 10 years use in EU.
Can promote amendments for Second Reading which allow traditional (e.g. 30-year) use from outside the EU with evidence from a competent authority.
Summary of ANH achievements in 2002 Mounted Brussels and UK-based campaign which helped to almost block FSD’s passage through Second (final) Reading in EU Parliament. Lobbied to ensure tabling of critical amendments on definition of a medicine, scope of directive and exclusions. All amendments successfully voted for at First Reading. Lobbied to help ensure major amendments were supported. Key amendment on non-EU traditional use was lost in pre-First Reading vote but successfully re-tabled by European Liberal Democrats at First Reading plenary. Although it again failed, it can be re-tabled at Second Reading.
A Ray of Light
2002 also saw some major revelations that showed that even the normally conservative medical establishment could at times warm to the notion of the importance of supplements. For example, in June 2002, two Harvard Medical School doctors, following a review of studies between 1966 and 2002 on the relationship between vitamin intake and various deficiency diseases concluded that all adults should take at least a multivitamin supplement daily . This may not sound like a major breakthrough to long-term vitamin and mineral advocates – but it most certainly was! They published their findings in the usually pro-pharmaceutical Journal of the American Medical Association which effectively reversed its long-term position which had up until this time been steadfastly anti-supplements.
Another important milestone, also from the other side of the Atlantic, was the US Food and Drug Administration’s (FDA) agreement in December 2002 to implement a ruling from three years ago (in favour of Pearson) that they had blatantly disregarded. This ruling allows manufacturers to make available more information to consumers about the effects of diet and dietary supplements on disease. Manufacturers will now be able to make health claims on products without requiring absolutely conclusive evidence, which can be very difficult to produce, without fear of being ravaged by the FDA! This is a substantial victory for health freedom – our thanks are owed primarily to Washington-based health freedom activist and attorney Jonathan Emord of Emord & Associates.
More hurdles
But it’s not all plain sailing! While some breakthroughs have occurred, including the European Parliament’s agreement to support ANH-backed amendments that would see many supplements excluded from the Pharmaceuticals Directive, there are still huge hurdles to be overcome. Of paramount importance is working to ensure that all the progress made in the EU during 2002 is made to stick. This is one of our key tasks for 2003 (see below).
One of the biggest challenges outside Europe – which cannot be separated from the processes at work within the EU – is the new dietary supplement evaluation scheme under development by the National Academy of Sciences in the US for future implementation by the FDA. This scheme, in its present form, has similarities with the European scheme in that it will cause, if allowed untroubled passage through Congress, slow strangulation of the innovative, non-pharmaceutical end of the dietary supplement industry. It will really force up the cost of innovation as all new ingredients will be pushed through an extremely onerous evaluation process that is not far removed from that required for drugs. Many non-pharmaceutical companies will simply not be able to afford these costs.
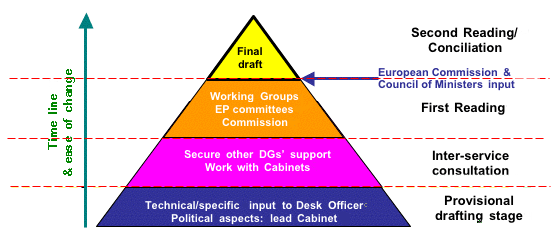
Figure 1. Outline of basic law-making procedure in the European Parliament
Global Campaign
We at ANH are working increasingly with contacts and experts in different parts of the world. We are now networking closely with contacts in Ireland, Netherlands, Denmark, Germany, France, Italy, the USA, Canada, Australia and New Zealand.
We believe it is essential to pull together the highest degree of multi-disciplinary expertise, in fields such as nutrition, pharmacology, law and public affairs, so that we can influence legislation so that it works in support rather than against public health. This is, if you like, the irony of the present campaign: here we are fighting a battle to protect consumer health, yet many legislators seem to be working ferociously in the opposite direction, trying to limit the availability of substances that are not only safe but are also critical to preventative health programmes.
Strategy for 2003
All of the progress made in 2002 will come unstuck if dialogue and interaction with the European Commission and the Council of Ministers (represented by health ministers from each EU member state) is not maintained to ensure the safe passage of key amendments affecting dietary supplements into EU law This is because these two organisations have the power to alter the parliamentary proposals, prior to Second Reading (Fig. 1). Although this sounds very undemocratic (which of course it is), it is justified in Brussels by the need to sort out irregularities or even contradictions in the amendments that emerge from First Reading in the European Parliament. For this reason it is absolutely essential that ANH and its counterparts in other parts of Europe continue working on these Directives.
Throughout 2003 it is essential that the ANH and other professional and consumer-based organisations supporting the availability of safe supplements are able to bring delegations to the European Parliament, the European Commission and the Council of Ministers. This must be done to ensure that the all the relevant science and laws in other countries are noted and considered in the EU law-making process. It is essential that the importance of treaties affecting public health policy and human rights of European citizens are fully appreciated. Governments must be helped to fully understand the importance of preventative health management – and they cannot be allowed to remove tools from the market that are invaluable for this process.
Good health and longevity!
Dr Rob Verkerk BSc, MSc, DIC, PhD
Executive Director
Alliance for Natural Health
e [email protected]
www.alliance-natural-health.org
tel +44 1252 371275
|
Donations for the ANH campaign are urgently needed!
Please send cheques and your contact details to: Alliance for Natural Health Mount Manor House, 16 The Mount, Guildford Surrey GU2 4HS, United Kingdom Alternatively payments can be made directly to the ANH bank account: Natwest Bank plc Guildford High Street Surrey, United Kingdom Sort code: 60-09-21 Account: 12765309 Swift code (for transactions from overseas): NWB KGB 2L |
© 2003 Alliance for Natural Health
1. Fairfield Kathleen MD, Fletcher Robert MD (2002) Vitamins for chronic disease prevention in adults: scientific review. Journal of the American Medical Association (JAMA), June 19, 287(23): 3116-26.
